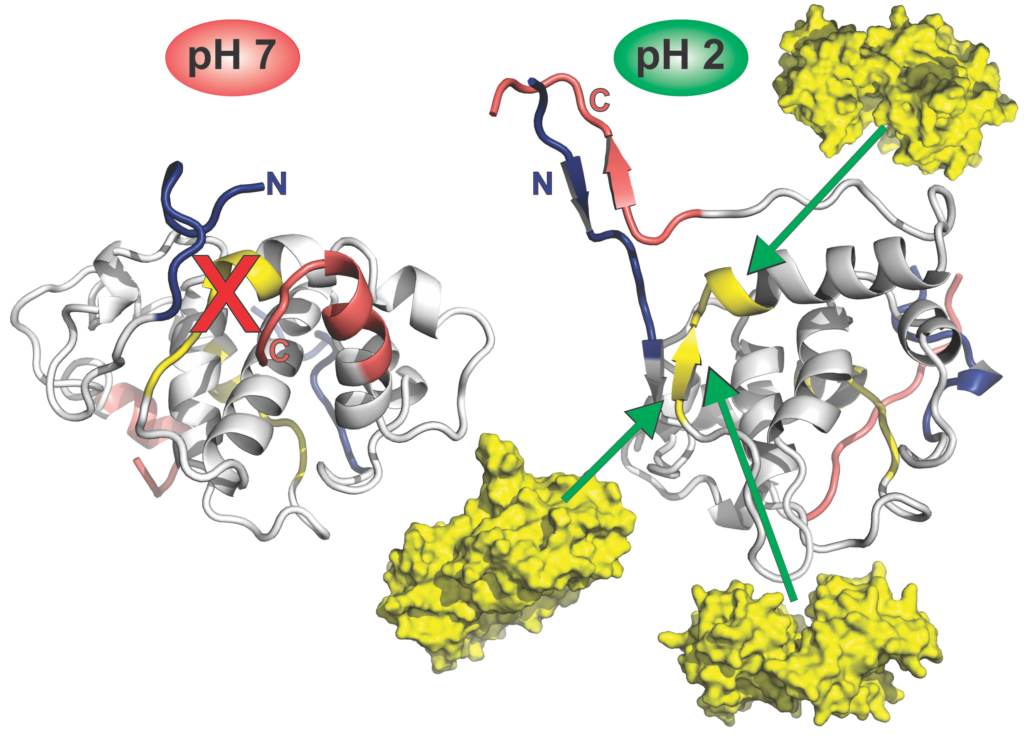
We were asked to provide the cover art for our latest publication in Biophysical Chemistry, collaboration with Dr Abrol. This will go on the cover of the September issue of the journal. The caption to accompany the image reads “HdeA is a chaperone protein activated at low pH. Through NMR experiments and MD simulations Pacheco et al. found that a hydrophobic client binding site is blocked at pH 7 by interactions with N- and C-terminal residues in the dimer. At pH 2, the HdeA chaperone becomes activated, in part, due to increased contacts between the N-and C-termini on each protomer, making the client binding site accessible.”
